Rabiopred Project : Media Centre
Welcome to Rabiopred Media Centre, a page regularly maintained, providing all relevant information and recent updates on the project, as well as downloadable material. For other questions, please contact us.
7-May-2018
FIRALIS S.A. launches the Biopred* Panel !
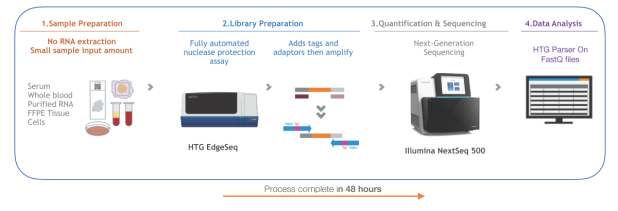
Biopred is an innovative RUO product, profiling with no RNA extraction,
thousands of mRNAs associated with inflammatory and autoimmune disorders.
The Biopred panel can be used for the development of personalized medicine applications such as diagnostic, prognostic and theragnostic tools for inflammatory and autoimmune disorders such as Rheumatoid arthritis, Ankylosing spondylitis, Sjögren's Syndrome, Systemic sclerosis, Systemic lupus erythematosus, Crohn’s disease, Ulcerative colitis, different forms of vasculitides, Inflammatory myopathies, Inflammatory cardiac diseases and many others.
The Biopred panel employs the innovative HTG EdgeSeq platform for targeted gene sequencing and NGS quantification, which offers a cost-effective solution for developing personalized medicine applications.
For more information, please contact sales@firalis.com
*Biopred has been developed thanks to the know-how generated during and by the Rabiopred Project, which is funded by the European Union, within the framework of Horizon 2020 SME Instrument program.
7-May-2018
Firalis SA, a provider of bioanalytical services, also biomarker-based products and services, signed a non‑exclusive license and supply agreement with HTG Molecular Diagnostics (Nasdaq:HTGM), a provider of instruments, reagents and services for molecular profiling applications, on 7th of May, 2018.
The agreement is a next step in the established relationship of both parties, following the successful adaptation of Firalis’ BIOPRED* research‑use assay to the HTG EdgeSeq technology. For more information:
*BIOPRED is a product realized in the framework of IMI BT-Cure (GA#115142) and Horizon 2020 Rabiopred (GA#666798) projects, funded by the European Commission.
10-Janvier-2018
Today, French Local Ethics Committee, CPP Nord-Ouest IV, has given a favorable opinion to start Healthy Volunteer study in the framework of RABIOPRED.
Tcland Expression SA has already signed a research service contract with "Le Centre Hospitalier Régional Universitaire de Lille" in order to execute this Study.
The study will be performed at CIC of Hôpital Calmette in Lille under the supervision of Pr. Dominique DEPLANQUE. The objectives of the study are (i) to characterize candidate biomarkers by assessing the effects of circadian rhythm, age, gender and by assessing their variability components, and (ii) to evaluate the effects of different sampling and handling procedures. The study has also received the regulatory approval from ANSM*.
* ANSM: Agence nationale de sécurité du médicament et des produits de santé
22-December-2016
Hopital Hautpierre, CHRU de Strasbourg
RABIOPRED consortium opens Hautepierre Hospital, Strasbourg for recruitment of Rheumatoid Arthritis patients who are naïve to biologics therapy. In a meeting held on December 22nd, 2017, study protocol and operational details were discussed in detail. It was agreed to start the clinical study as soon as possible.
The Rheumatology department of the Strasbourg University Hospital serves as a reference center of care for various auto-immune and rheumatologic disorders. The center is coordinating a national cohort of RA patients and has a proven track record of internationally recognized research achievement in the field. In this project, Prof. Jean Sibilia and Prof. Jacques-Eric Gottenberg are participating as Principal Investigators. Both are practicing physician involved in a number of clinical studies and trials. Prof. Sibilia is also the Dean of the Faculty of Medicine of the University of Strasbourg and Head of the Rheumatology Unit, while Prof. Gottenberg is a leading KOL (key opinion leader) in the field of rheumatoid diseases and is intensively involved in the development of personalized care for RA patients.
21-November-2016
Tcland Expression S.A., coordinator of the Rabiopred Project, signed a contract with Koehler eClinical Gmbh to perform Electronic Data Capture of the PoP* clinical study.
KOEHLER eClinical is an independent owner-operated contract research organization with over 20 years experience in providing clinical research services to the biopharmaceutical industry and life science organizations.
* PoP: Proof-Of-Performance
18-November-2016
Tc Land Expression SA signed a research service contract with "Le Centre Hospitalier Régional Universitaire de Lille" in order to execute a Healthy Volunteer Study.
The study will be performed at CIC 1403 Hôpital Calmette, Boulevard du Pr Leclercq 59037 Lille Cedex within the supervision of Pr. Dominique DEPLANQUE, the primary
investigator. The objectives of the study are (i) to evaluate the effects of different sampling and handling procedures and (ii) to characterize candidate biomarkers by assessing the effects of
circadian rhythm, age, gender and by assessing their variability components. The study has received the regulatory approval from ANSM* and a positive opinion from Ethics committee “CPP
Nord-Ouest IV”.
* ANSM: Agence nationale de sécurité du médicament et des produits de santé
12-September-2016
"Les Echos", one of the most prestigious financial journals of reference, published an article on the collaboration agreement of Firalis Group with HTG Molecular Diagnostics that combined efforts within the Rabiopred Project to develop a theranostic tool to predict response to anti‑TNFα therapies for Rheumatoid Arthritis (RA).
For the complete original article, click on the link below :
« Firalis prédit l’efficacité des thérapies contre la polyarthrite rhumatoïde »
26-July-2016
Molecular Diagnostic Department of Firalis and TcLand Expression, leading Rabiopred project that aims to develop personalized treatment strategies for rheumatoid arthritis patients, using miRNA profiling, realised a webinar on the thematic of "New Tools for Biomarker Discovery Promise Personalized Treatment for Rheumatoid Arthritis". During the webinar, Dr. Eric Schordan gave details of the study and plans to validate the Rabiopred panel in a multicentric prospective clinical study of 600 patients.
Access the webinar and even upload the complete recording here:
7-July-2016
HTG and Firalis Agreed to Develop a Theranostic Tool to Predict Response to Anti TNFα Therapies for RAHTG and Firalis Agreed to Develop a Theranostic Tool to Predict Response to Anti TNFα Therapies for RA.
Firalis SA,
a provider of
bioanalytical services and biomarker-based products to biopharma, biotechnology companies and academia, a participant of RABIOPRED
Project (coordinated by Tc Land Expresison SA), reached to an agreement with HTG Molecular Diagnostics, Inc. (Nasdaq: HTGM; “HTG”), a provider of instruments and reagents for
molecular profiling applications; to develop a next‑generation sequencing (NGS)-based theranostic tool to identify likely non-responders from responders to anti‑TNFα therapy for rheumatoid
arthritis (RA).
RA is a chronic inflammatory disease that affects millions of people in the United States and western Europe. TNFα-inhibiting therapies, the blockbusters representing a multibillion dollar market, have shown a major breakthrough in the treatment of RA. However, these drugs are found to lack efficacy in an estimated 30 to 40 % of patients (non-responders). The RABIOPRED tool aims to identify the non-responders to improve patient management. Under the agreement, HTG will supply instrumentation and reagents to support Firalis’ development, validation and clinical deployment of RABIOPRED, a BIOmarker assay to PREDict treatment response in RA, using the NGS-based HTG EdgeSeq system.
“We are pleased to expand our relationship through this personalized medicine initiative,” said TJ Johnson, President and CEO of HTG.
“Millions of RA patients are treated with TNFα-inhibiting agents but the clinicians currently are unable to predict patient response to these expensive biologicals,” said Hüseyin Firat, President & CEO of Firalis, and Tc Lad Expression, latter being the coordinator of RABIOPRED Project. “We believe the ability to predict response to anti‑TNFα treatment would greatly improve clinical decision-making, improve health outcomes for RA patients, and contribute to cost reduction and sustainability of the health care system.”
About HTG:
Headquartered in Tucson, Arizona, HTG’s mission is to empower precision medicine at the local level. In 2013, the company commercialized its HTG Edge instrument platform and a portfolio of RNA assays that leverage HTG’s proprietary nuclease protection chemistry. HTG’s product offerings have since expanded to include its HTG EdgeSeq product line, which automates sample and targeted library preparation for next-generation sequencing.
For additional information: www.htgmolecular.com
February 2016
RABIOPRED Team got a first Regulatory Advice from EMA (European Medicines Agency) on an informal meeting.
Rabiopred project team held a first informal meeting with the European Medicines Agency (EMA) in London to obtain an initial feedback on the project. Rabiopred project aims to address the unmet need
for biomarkers that can predict non-response to anti-TNFα therapies used as Rheumatoid Arthritis (RA) treatment. These biomarkers could thus guide the therapeutic decision-making.
Thanks to input received in this meeting, Rabiopred team has fine-tuned its clinical study.
Subsequently, the Rabiopred consortium is planning to apply for a qualification procedure at EMA and FDA in order to get the required regulatory approvals for the use of the Rabiopred biomarkers in
clinics.
7-Jul-2015
RABIOPRED Project launched with a Consortium Kick-off Meeting
In coordination of Tc Land Expression S.A. and with participation of all clinical centers (mentioned here in detail) represented by their top management, RABIOPRED project is launched via an excellent kick-off meeting. The meeting is held in the administrative site of Tc Land in Huningue, at the triple border of France, Switzerland (Basel Region) and Germany (Freiburg). Thanks to this central position of the Tc Land and Firalis facilities, the participation to the kick-off meeting was complete. All parties have represented their institutes, specialities and capabilities by focused and uniformed presentations.
Firalis SAS, the parent company of Tc Land and a project partner (as third linked party) and other stakeholders such as candidate consultants and outsourcing partners were also present at the meeting.
Next step is to have further discussions with each of the stakeholders and clinical centers in particular, in order to prepare the clinical trial in a best possbile configuration.